






References: 1. Nurofen for Children Strawberry 3 months to 12 years. Oral Suspension. Summary of Product Characteristics. April 2021. Accessed August 2023. 2. Barbagallo M & Saceerdote P. Minerva Paediatr.
2019;71(1):82–99. 3. Kelley MT et al. Clin Pharmacol Ther. 1992;52(2):181–189. 4. Pelen F et al. Ann Pediatr. 1998;45:719–728.
Essential Information
Nurofen for Children Strawberry 3 months to 12 years
PL00063/0666
Active Ingredient(s): Ibuprofen 100 mg/5 ml (2.0%w/v).
Indications: Prescription only: For symptomatic treatment of Juvenile Rheumatoid Arthritis. Prescription and OTC: For the fast and effective reduction of fever, including post immunisation pyrexia and the fast and effective relief of the symptoms of colds and influenza and mild to moderate pain, such as a sore throat, teething pain, toothache, earache, headache, minor aches and sprains. Dosage and Administration: For oral administration. For short-term use only. The lowest effective dose should be used for the shortest duration necessary to relieve symptoms For patients with sensitive stomachs the product can be taken with or after food. For children between 3 months and 12 years of age. The recommended daily dose is 20–30 mg per kg of body weight, divided into equal doses, with dosing intervals of 6-8 hours (see the pack for detail). Leave at least 4 hours between doses. Do not take more than the recommended dose in 24 hours. Do not exceed the recommended dose. Not suitable for children under 3 months of age unless advised by a doctor. Do not use this product in children weighing less than 5 kg. For infants aged 3-6 months ask for medical advice if symptoms worsen or not later than 24 hours if symptoms persist. If in children from 6 months this medicinal product is required for more than 3 days, or if symptoms worsen, consult a doctor. For children under 6 months ask for medical advice after 24 hours use (3 doses) if the symptoms persist. Special patient groups: children with Juvenile Rheumatoid Arthritis: The usual daily dose of 30 to 40 mg per kg of body weight, in three to four divided doses may be taken. Post-immunisation pyrexia: One 2.5 ml dose followed by one further 2.5 ml dose 6 hours later if necessary. Do not exceed two 2.5ml doses in 24 hours. If the fever is not reduced, consult a doctor. Contraindication: Hypersensitivity to ibuprofen or to any of the excipients. History of hypersensitivity reactions in response to ibuprofen, acetylsalicylic acid or other NSAIDs. Active or a history of recurrent peptic ulcer/haemorrhage (two or more distinct episodes of proven ulceration or bleeding). History of gastrointestinal bleeding or perforation, related to previous NSAIDs therapy. Severe hepatic failure, renal failure or heart failure. Last trimester of pregnancy. Special warnings and Precautions for use: Do not give this product if the child is under 3 months old or weighs less than 5 kg; has (or has had two or more episodes of) a stomach ulcer, perforation or bleeding; is allergic to ibuprofen or any other ingredient of the product, aspirin or other related painkillers, or fructose; is taking other NSAID painkillers, or aspirin with a daily dose above 75mg. Consult a doctor or pharmacist before use for someone who has or had asthma, diabetes, high cholesterol, high blood pressure, a stroke, heart, liver, kidney or bowel problems; is dehydrated; has chicken pox; smokers; pregnant, breastfeeding or women trying to get pregnant; elderly. Acute generalised exanthematous pustulosis (AGEP) has been reported in relation to ibuprofen-containing products. This medicine can mask symptoms of infection, which may lead to delayed initiation of appropriate treatment and thereby worsening the outcome of the infection. This has been observed in bacterial community acquired pneumonia and bacterial complications to varicella. You must consult a doctor if symptoms persist or worsen, or if the medicine is needed for more than 24h for a child of 3 to 6 months, or for more than 3 days for a child over 6 months. Contains Maltitol Liquid. Fertility, Pregnancy and Lactation: Pregnancy: Inhibition of prostaglandin synthesis may adversely affect the pregnancy and/or the embryo/foetal development. Data from epidemiological studies suggest an increased risk of miscarriage, cardiac malformation, and gastroschisis after using a prostaglandin synthesis inhibitor in early pregnancy. The absolute risk for cardiovascular malformation increased from less than 1% to approximately 1.5 %. The risk is believed to increase with dose and duration of therapy. In animals, administration of a prostaglandin synthesis inhibitor has been shown to result in increased pre- and post-implantation loss and embryo-foetal lethality. In addition, increased incidences of various malformations, including cardiovascular, have been reported in animals given a prostaglandin synthesis inhibitor during the organogenetic period. During the first and second trimester of pregnancy, Nurofen for Children should not be given unless clearly necessary. If Nurofen for Children is used by a woman attempting to conceive, or during the first and second trimester of pregnancy, the dose should be kept as low and duration of treatment as short as possible. During the third trimester of pregnancy, all prostaglandin synthesis inhibitors may expose the foetus to: - cardiopulmonary toxicity (with premature closure of the ductus arteriosus and pulmonary hypertension); - renal dysfunction, which may progress to renal failure with oligo-hydroamniosis; the mother and the neonate, at the end of pregnancy, to: - possible prolongation of bleeding time, an anti-aggregating effect which may occur even at very low doses. - inhibition of uterine contractions resulting in delayed or prolonged labour. Consequently, Nurofen for Children is contraindicated during the third trimester of pregnancy. Breast-feeding: In limited studies, ibuprofen appears in the breast milk in very low concentration and is unlikely to affect the breast fed infant adversely. Fertility: There is limited evidence that drugs which inhibit cyclo-oxygenase/ prostaglandin synthesis may cause impairment of female fertility by an effect on ovulation. This is reversible upon withdrawal of treatment. Side effects: The following list of adverse effects relates to those experienced with ibuprofen at OTC doses (maximum 1200 mg ibuprofen per day), in short-term use. In the treatment of chronic conditions, under long-term treatment, additional adverse events may occur. Adverse events which have been associated with ibuprofen are given below, tabulated by system organ class and frequency. Frequencies are defined as: very common (≥1/10), common (≥1/100 and <1/10), uncommon (≥1/1000 and <1/100), rare (≥1/10,000 and <1/1000), very rare (< 1/10,000) and not known (cannot be estimated from the available data). Within each frequency grouping, adverse events are presented in order of decreasing seriousness. The most commonly observed adverse events are gastrointestinal in nature.
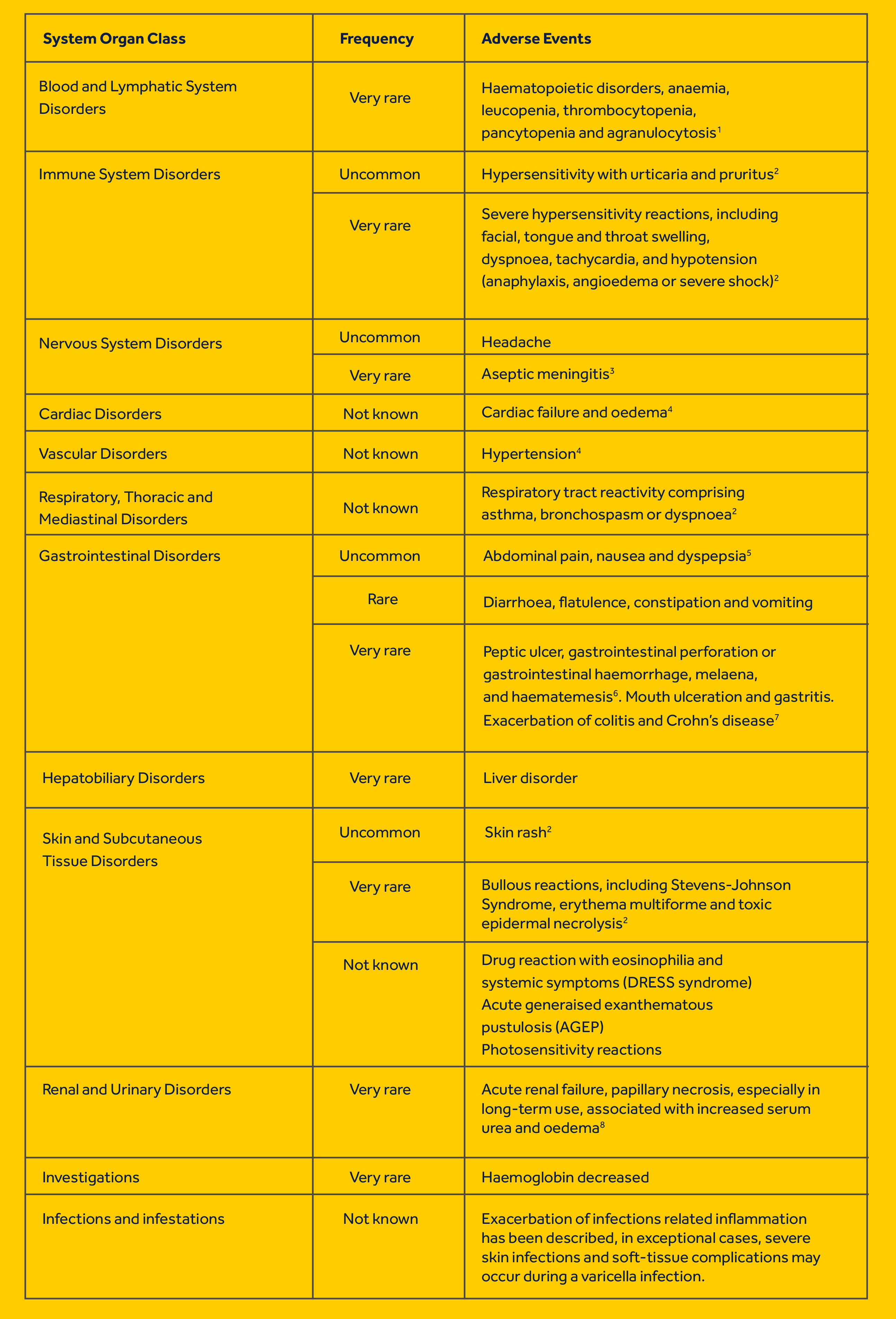
Description of Selected Adverse Reactions
1 First signs are fever, sore throat, superficial mouth ulcers, flu-like symptoms, severe exhaustion, unexplained bleeding and bruising.
2 Hypersensitivity reactions: These may consist of (a) non-specific allergic reactions and anaphylaxis, (b) respiratory tract reactivity, including asthma, aggravated asthma,
bronchospasm, and dyspnoea, or (c) various skin reactions, including pruritus, urticaria, purpura, angioedema and, more rarely, exfoliative and bullous dermatoses, including toxic epidermal necrolysis, Stevens-Johnson Syndrome and erythema multiforme.
3 The pathogenic mechanism of drug-Induced aseptic meningitis is not fully understood. However, the available data on NSAID-related aseptic meningitis points to a hypersensitivity reaction (due to a temporal relationship with drug intake, and disappearance of symptoms after drug discontinuation). Of note, single cases of symptoms of aseptic meningitis (such as stiff neck, headache, nausea, vomiting, fever or disorientation) have been observed during treatment with ibuprofen in patients with existing auto-immune disorders (such as systemic lupus erythematosus and mixed connective tissue disease).
4 Clinical trial and epidemiological data suggest that use of ibuprofen (particularly at high doses 2400 mg daily) and in long-term treatment may be associated with a small increased risk of arterial thrombotic events (e.g. myocardial infarction or stroke), (see section 4.4).
5 The adverse events observed most often are gastrointestinal in nature.
6 Sometimes fatal.
7 See section 4.4.
8 Especially in long-term use, associated with increased serum urea and oedema. Also includes papillary necrosis.
Legal classification: P. Licence Holder: Reckitt Benckiser Healthcare (UK) Limited HU8 7DS.
Product licence Numbers: PL 00063/0666. Price: £8.15, 200 ml. Last revised: 30/April/2021 For full information refer to: https://www.medicines.org.uk/emc/product/393/smpc
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for the MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Reckitt Benckiser Healthcare (UK) ltd on: 0333 200 5345